Hydrogenation of vegetable oil
Company news / Chat on line / Give me a price / Date: June 23, 2016
Hydrogenation of vegetable oil, why?
Vegetable oils contain a mix of saturated, monounsaturated, and polyunsaturated fatty acids. The mono- and polyunsaturated fatty acids have double bonds, all in the normal “cis” formation. These bonds can easily be broken down by oxygen. This produces compounds that make the oil rancid. Rancidity produces off-flavors in foods.
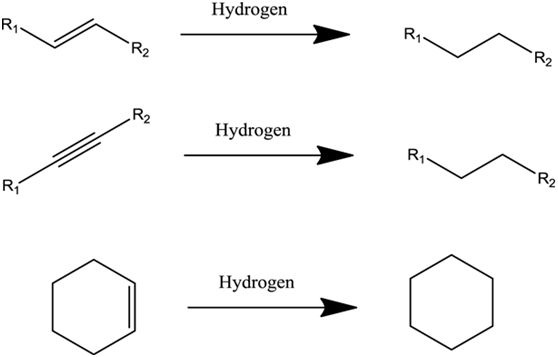
Hydrogenated vegetable oils chemical process
Vegetable oils contain a mix of saturated, monounsaturated, and polyunsaturated fatty acids. The mono- and polyunsaturated fatty acids have double bonds, all in the normal “cis” formation. These bonds can easily be broken down by oxygen. This produces compounds that make the oil rancid. Rancidity produces off-flavors in foods.

Hydrogenated vegetable oils chemical process
To control this, food manufacturers use hydrogenated vegetable oils. These are not as likely to break down and will produce a product with a longer shelf life. Hydrogenation is a chemical process that adds hydrogen atoms to the available double bonds in the vegetable oil. As the degree of hydrogenation increases, the amount of saturated fats increases and mono and polyunsaturated fats decrease. Completely hydrogenated fat is solid at room temperature. Moderately hydrogenated fats are liquid at room temperature and contain more saturated fatty acids than the original oil.
Hydrogenation of vegetable oil benefits a lot to shelf life, but hydrogenation also brings trans fatty acid, which is bad for human health. We should pay more attention to the content of hydrogenated vegetable oil.
Hydrogenation of vegetable oil benefits a lot to shelf life, but hydrogenation also brings trans fatty acid, which is bad for human health. We should pay more attention to the content of hydrogenated vegetable oil.
contact us
- QDo you want to buy machine?
- Yes, I want to buy machine.
- No, I want to learn more in advance.
- QWhat oil seeds do you want to process?
- Palm fruit
- Palm kernel/nut
- Peanut/Groundnut
- Soybean/Soya bean
- Sunflower seed
- Cottonseed
- Rapeseed/Canola
- Dried coconut
- Rice bran
- Corn germ
- More than two oilseeds:
- Other:
- QHow many tons palm fruit bunches will you process per day?
- 1-10 tons per day
- 10-30 tons per day
- 30-50 tons per day
- 50-100 tons per day
- QWhat machine do you want?
- Palm oil presser
- Other single machine (thresher, clarification tank, vibrating screen, filter...)
- Palm oil pressing line (from FFB to crude oil)
- Palm oil refining line (to produce refined, bleached, deodorized oil)
- Palm oil bottling / filling line
- QWhat machine do you want?
- Palm oil pressing line (from FFB to crude oil)
- Palm oil refining line (to produce refined, bleached, deodorized oil)
- Palm oil bottling / filling line
- QHow many tons oil seeds will you process per day?
- 1-20 tons per day
- 20-50 tons per day
- 50-100 tons per day
- QWhat machine do you want?
- Oil presser
- Other single machine (cracker, crusher, roaster, filter...)
- Oil pressing line (from seeds to crude oil)
- Oil refining line (to produce refined, bleached, deodorized oil)
- Oil bottling / filling line
- QWhat machine do you want?
- Oil presser
- Oil pressing line (from seeds to crude oil)
- Oil solvent extraction line
- Oil refining line (to produce refined, bleached, deodorized oil)
- Oil bottling / filling line
- QWhat machine do you want?
- Oil pressing line (from seeds to crude oil)
- Oil solvent extraction line
- Oil refining line (to produce refined, bleached, deodorized oil)
- Oil bottling / filling line